The Effect of Hurricane Hermine on Black Sea Bass
The following article first appeared on the Research Blog for Dr. Neil Hammerschlag’s Shark Research and Conservation (SRC) Lab website at the University of Miami’s Rosenstiel School of Marine and Atmospheric Science. To learn more about SRC, visit here: http://sharkresearch.rsmas.miami.edu/, or to learn more about the University’s marine science school, please click here: http://rsmas.miami.edu/.
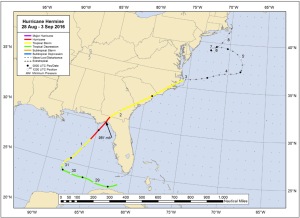
Figure 1: Best Track Positions for Hurricane Hermine. This map is a composite of the best predicted tracks of Hurricane Hermine between August 28th and September 3rd, 2016. Offshore of western Florida, it transformed from a tropical storm to a hurricane, making landfall as a category one hurricane, and then transitioning back into a tropical storm as it made its way across the state into the eastern waters off Maryland. (Source: Berg 2017).
In September of 2016, Hurricane Hermine struck Florida as a category one hurricane and then migrated through Georgia, South Carolina, North Carolina, and then to offshore Maryland. According to the National Oceanic and Atmospheric Administration (NOAA) National Centers for Environmental Information (NCEI), Hermine’s damage “totaled around $550 million, with a 90% confidence interval of +/- $150 million” and demolished 1,600 homes and businesses (Berg 2017). But how did it affect offshore fish populations? Researchers from the University of Maryland designed an experiment to find out.
Four months before Hermine hit Florida, 45 black sea bass were acoustically tagged and acoustic receivers were moored in the shelf waters of three different sites off Maryland; a northern, middle, and southern site. Rash winds of Hurricane Hermine caused destratification, “a process in which the air or water is mixed in order to eliminate stratified layers of temperature, plant, or animal life,” in the water column of the Mid-Atlantic Bight. Due to this disarrangement, temperatures of northern and middle experimental sites rose 10 degrees Celsius in just ten hours creating an unsuitable environment for living organisms and, thus, either migration or death of the black sea bass was expected.
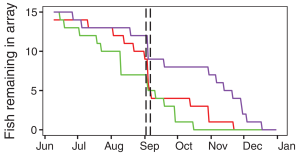
Figure 2: Black Sea Bass Population Size, Summer 2016. This graph exhibits the decay in population size of black sea bass between the three experimental sites. The two vertical, black, hash-marked lines indicate September 2nd – 6th. All three experimental sites showed a decline in black sea bass populations and by January of 2017, all three populations had diminished completely. (Source: Secor et al. 2017).
Researchers discovered that 40% of the sea bass populations had evacuated the experimental sites in search of a more suitable habitat and any that stayed behind exhibited decreased activity levels showing that there were large behavioral changes due to the increased temperatures. Evacuation was found to be highest in the northern and southern sites and lower in the middle site and in most cases, migration was permanent. Although some recovery was indicated in the two weeks following Hermine, water column stratification and black sea bass population sizes did not return to normal (Secor et al. 2017).
Although hurricanes are just one of the factors contributing to the emigration of fish species, as our planet continues to warm, hurricanes are predicted to become more intense and more frequent potentially leading to even larger emigration phenomena which would ostensibly take a large toll on the fishing industry. According to the Fisheries Economics of the U.S. 2011 report, recreational fishing in the South Atlantic generates 52,000 jobs and adds $3 billion to the United States’ GDP (Back in Black). Due to their importance to our economy and the threats that they face, it will be imperative to monitor black sea bass and fisheries to ensure that measures are being taken to stabilize the economy when their performances decline.
Works cited
Berg, Robbie. “Hurricane Hermine.” National Hurricane Center Tropical Cyclone Report, 30 Jan. 2017.
Secor, D. H., Zhang, F., O’Brien, M. H., & Li, M. (2018). Ocean destratification and fish evacuation caused by a Mid-Atlantic tropical storm. ICES Journal of Marine Science.
USA Department of Commerce, 27 Sept. 2013. “Back in Black: Black Sea Bass Stock Is Rebuilt.” Accessed from: www.commerce.gov/news/blog/2013/09/back-black-black-sea-bass-stock-rebuilt.